Quality Management & Instrumentation Division
Quality Management & Instrumentation Division is a central facility for the Institute which provides scientific data from various sophisticated analytical instruments. The Division provides services in four major domains: Instrumentation, Quality Control & Quality Assurance, Drug Testing Lab and cGMP facility.
Instrumentation: Spectrometry, chromatography and microscopy form the three major units of the division which help in structure elucidation and characterization of various compounds of natural as well as synthetic origins. The Division is equipped with state of the art analytical facility consisting of High Resolution Mass Spectrometer, LC-MS, NMRs, GC-MS, HPLC, SEM & TEM, Confocal Microscope besides many other commonly used scientific equipment. Major S&T data of the Institute is generated in Instrumentation. Besides catering to requirements of scientists and students of IIIM, Instrumentation also provides services to national academia, research organizations and industry on chargeable basis.
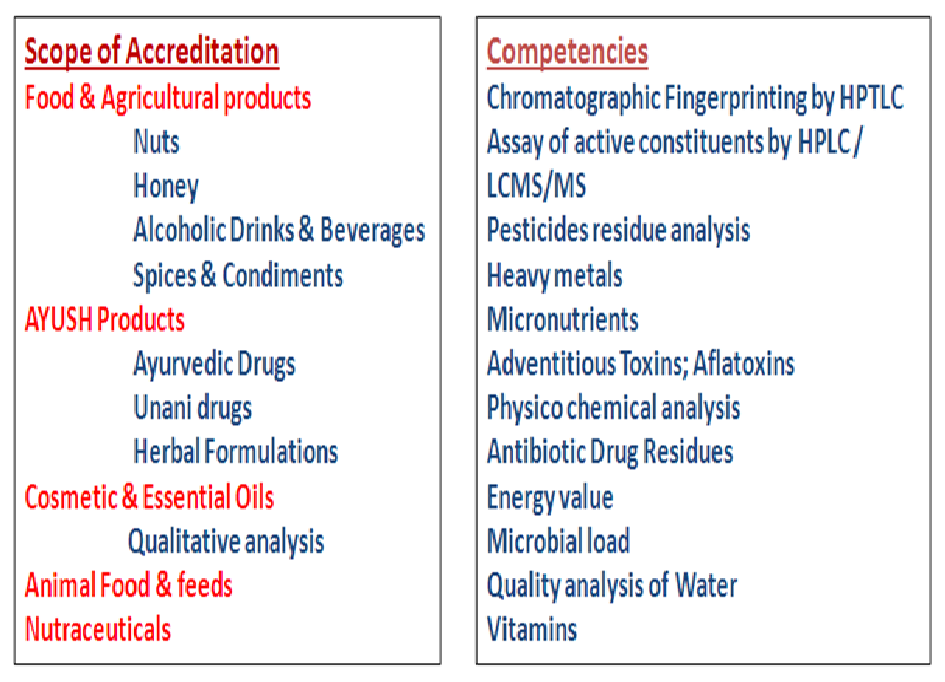
Quality Control & Quality Assurance: QCQA is dedicated to render analytical services of highest quality meeting the requirement of the standard ISO/ IEC 17025:2017 and other criteria’s of NABL. It is the first notified Food Testing Laboratory in UT of J & K approved by GOI, Ministry of Health and Family Welfare under Section 43 (1) of FSS Act, 2006 for food commodities such as Nuts, Honey and Nutraceuticals. The scope of QCQA includes testing of food & agricultural products, Ayush products, cosmetics & essential oils, Animal foods & feeds and Nutraceuticals.


Drug Testing Lab: It’s an allopathic drug testing facility which provides services to various pharmaceutical companies and government organization on chargeable basis. The facility has been recognized by Food & Drug Control Organization of J&K. State-of-the-art analytical instruments have been installed for testing various parameters of allopathic drugs.
Chemical Engineering & c-GMP: Chemical Engineering Division of CSIR-IIIM, Jammu is engaged in process development (Lab Scale) and scale-up of laboratory processes on a pilot plant under Non-GMP parameters. It is involved in commercial-scale steam distillation of aromatic crops for the production of essential oils and value-added products. The facility has walk-in-stability chambers for stability studies on real-life conditions and accelerated conditions as per the ICH guidelines for NCE/IND molecules.
- Vision: To acquire and maintain latest instrumental analytical and production facilities for providing cutting edge techniques for generation of scientific analytical data and products.
- Mission: To generate accurate and precise scientific data for scientists and students for carrying out research as per the mandate of the Institute. Also, to provide internationally acceptable data to commercial establishments.
- State-of-the-art equipments for various type of chemical analysis of samples of natural as well as synthetic origin.
- Spectroscopic, chromatographic and microscopic analysis of samples as per the requirements of the researcher.
- Chemistry Manufacturing & Control of Botanical Raw Material, Botanical Drug Extract and Botanical product / formulations.
- Quality Control, Evaluation and Identification for characteristic marker in Medicinal & Aromatic Plants.
- Quality Control and Assurance for authentication of Indian System of Medicine products and formulations.
- Qualitative analysis of Essential oils.
- Physicochemical analysis of Water.
- Analysis of micronutrients & heavy metals.
- Analysis of residual pesticides.
- Analysis of microbial load / pathogens in food and water.
- Process Development and scale-up of chemical processes from laboratory to pilot scale.
- State-of-the-art cGMP extraction plant
- Extraction plant capacity of around 15-20 kg of material per batch.
- Process upscaling from 100 g to 15/20 kg scale with reduced steps & solvents.
List of research facilities/ infrastructure available
Following analytical instruments available in the department.
| Name of equipment | Make | Installation Year | |
| 1. | NMR 400 MHz | Bruker | 2007 |
| 2. | NMR 500 MHz | Bruker | 2005 |
| 3. | NMR 400 MHz with autosampler | Bruker | 2020 |
| 4. | High Resolution Mass Spectrometer | Agilent | 2011 |
| 5. | LC-MS/MS | Shimadzu | 2012 |
| 6. | GC-MS/MS | Shimadzu | 2019 |
| 7. | Analytical HPLC | Shimadzu | 2014 |
| 8. | Prep HPLC | Waters | 2000 |
| 9. | UV-Vis spectrophotometer | Shimadzu | 2012 |
| 10. | FT-IR Spectrophotometer | Perkin Elmer | 2012 |
| 11. | CHN Analyser | Elementar | 2012 |
| 12. | Polarimeter | Perkin Elmer | 1992 |
| 13. | Circular diochroism spectrometer | Jasco | 2016 |
| 14. | Transmission Electron Microscope | Jeol | 2017 |
| 15. | Scanning Electron Microscope | Jeol | 2017 |
| 16. | Confocal Microscope | Olympus | 2000 |
| 17. | High-throughput Imaging System | Yokogawa | 2019 |
| 18. | Liquid nitrogen Plant | StirLin | 2000 |
| 19. | Liquid Nitrogen Plant | Wirac | 2015 |
QCQA has following State of Art facilities
| S.No | Instrument | Analysis | ||
| 1 | LC-MS/MS Triple Quad | Quantitative Analysis | Heavy Metals | |
| 2 | ICP-MS | Heavy Metals | ||
| 3 | HPLC Nexera | Qualitative & quantitative analysis | ||
| 4 | HPTLC | Chemical fingerprinting | ||
| 5 | Kjeldahl apparatus | Estimation of Nitrogen |
Facilities available in DTL
|
- STS0003 – Testing & Calibration (STS0003)
- Cannabis germplasm evaluation and its phytocannabinoids study
- CSIR-Nutraceuticals and Nutritionals Mission HCP0019
- CSIR-Aroma mission-HCP0007
- CSIR-Phytopharmaceuticals mission HCP00010
- Development of processes for Active Pharmaceutical Ingredients towards COVID 19- HCP-29
- (CNP-1313) – Herbal Dengue Product: Scientific and Technical development at CSIR-IIIM,Jammu
- Sickel-Cell anemia mission
- chemical repository project
- Quality Control, Quality Assurance and Chemistry Manufacturing and Control of drugs : MLP-7001 & STS 0006.
- Phytopharmaceutical development of Ficus semicordata as per regulatory guidelines of DCGI: GAP 2180.
- Development of Boswellia serrata based Phytopharmaceutical Drug for Osteoarthritis.
- CBD-THC Enriched Phytopharmaceutical Drug for Cancer Pain management
| Authors | Title | Journal |
| R.V. Singh H. Sharma A. Ganjoo A. Kumar V. Babu | Novel amidase catalysed process for the synthesis of vorinostat drug | Journal of Applied Microbiology, 2020 |
| Sougata Sarkar, Pooja Saraswat, Amit Kumar | Allelopathy as a tool to influence essential oil quality profile of mentha piperita | Journal Of Medicinal And Aromatic Plant Sciences, 2020 |
| Rather G, Sharma A, Kumar A , Lattoo S. et al | “Molecular characterization and overexpression analyses of secologanin synthase to understand the regulation of camptothecin biosynthesis in Nothapodytes nimmoniana (Graham) Mabb | Protoplasma,2019 |
| Singh A, Kumar A , Singh G.D. et al | Rohitukine inhibits NF-κB activation induced by LPS and other inflammatory agents | International Immunopharmacology,2019 |
| Inshad A. Khan, Amarinder Singh, Durga P. Mindala, Samdarshi Meena, Bhavana Vij, Arvind K. Yadav, Sumit Roy, Utpal Nandi, Anil K. Katare, Sundeep Jaglan, Deepika Singh, Ajai P. Gupta, Prasoon Gupta, Surjeet Singh, Manoj K. Tikko, Gurdarshan Singh, Ram A. Vishwakarma. | “Preclinical development of gastro-protective botanical candidate from Woodfordia fruticosa: Chemical standardization, Efficacy, Pharmacokinetics and Safety pharmacology” | Journal of Ethnopharmacology (SCI) (IF 3.690) |
| Anil Kumar Katare, Bikarma Singh, Pooja Shukla, Sandeep Gupta, Bishander Singh, Kavya Yalamanchili, Nitin Kulshrestha, Rajendra Bhanwaria, Ashok Kumar Sharma, Sarita Sharma, Sneha, Durga Prasad Mindala, Sumit Roy, Rahul Kalgotra | “Rapid determination and optimisation of berberine from Himalayan Berberis lycium by soxhlet apparatus using CCD-RSM and its quality control as a potential candidate for COVID-19” | Natural Product Research (SCI) (IF 2.20) |
| Singh A, Thatikonda T, Kumar A , Wazir P, Singh G.D. et al | Determination of ZSTK474, a novel Pan PI3K inhibitor in mouse plasma by LC–MS/MS and its application to Pharmacokinetics | J. Pharm. Biomed. Anal.,2018 |
| Varun P. Singh, Anup S. Pathania, Manoj Kushwaha, Samsher Singh, Vandana Sharma, Fayaz Malik, Inshad Khan, Anil Kumar, Deepika Singh*, Ram A. Vishwakarma* | 14-Residue Peptaibol Velutibol A from Trichoderma velutinum: Its structural and cytotoxic evaluation. | RSC Advances, 2020, 10, 31233-31242. |
| Arora D, Kumar A , Gupta P, Chashoo G, Jaglan S. | Preparation, characterization and cytotoxic evaluation of bovine serum albumin nanoparticles encapsulating 5-methylmellein: A secondary metabolite isolated from Xylaria psidii | Bioorganic Med Chem. Lett., 2017 |
| Wani ZA, Kumar A, Sultan P, Bindu K, Riyaz-Ul-Hassan S, Ashraf N | ’’Mortierella alpina (CS10E4), an oleaginous fungal endophyte of Crocus sativus L. enhances apocarotenoid biosynthesis and stress tolerance in host plant | Sci. Rep. 2017 |
| Vikas Kumar, Sonali Bharate, M. Gupta, S. G. Gandhi, Deepika Singh, Sundeep Jaglan, A. Kumar, Ram A Vishwakarma, Sandip B Bharate | Evaluation of rohitukine-enriched fraction of Dysoxylum binecteriferum Hook. F. (Leaves) as Anti-arthritic Phytopharmaceutical Candidate: Chemical Standardization, In-vivo validation, Formulation development and Oral Pharmacokinetics. | Journal of Ethnopharmacology, 2020, 254, 112758. |
| Varun P Singh, Richa Sharma, Vandana Sharma, Chand Raina, Kamal K Kapoor, Anil Kumar, Asha Chaubey, Deepika Singh*, Ram A Vishwakarma* | Isolation of depsipeptides and optimization for enhanced production of valinomycin from the North-Western Himalayan cold desert strain Streptomyces lavendulae | Journal of Antibiotics, 2019, 72, 617-624 |
| R. Anand, N. Verma, D.K.Gupta, S.C. Puri, G. Handa and G.N. Qazi | Comparison of extraction techniques for extraction of bioactive molecules from Hypericum perforatum L. plant. | Journal of Chromatographic Science, Vol 43, Nov/Dec 2005 |
| R. Anand, S.C.Puri, N. Verma, G. Handa, R.K.Khajuria, V.K.Gupta, O.P. Suri and G.N.Qazi | A simple and reliable semi-preparative high performance liquid chromatography technique for isolation of marker-grade hyperforin from Hypericum perforatum L. extract. | Journal of Chromatographic Science, Vol 41, No. 8, Sept 2003 |
| Suphla Gupta, Pankaj Pandotra, Gandhi Ram, Rajneesh Anand, Ajai Prakash Gupta, Mohd. Kashif Hussain, Yashbir Singh Bedi and Gopal Rao Mallavarapu | Comparison of monoterpenoid-rich essential oil from the Rhizome of Zingiber officinale from North Western Himalayas. | Natural Product Communications, 2011, Vol. 6, No. 1, 93-96, |
| Inventors/ Contributors | Title | Description | Year |
| Vikash Babu, Rahul Vikram Singh, Hitesh Sharma, Amit Kumar, Shaista Sultan, Varun Pratap Singh, Bhahwal Ali Shah, Parvinder Pal Singh | Enzymatic Process for the Preparation of Vorinostat | IN 201911047261 | 2019 |
| G.N.Qazi, S.C.Puri, A. Braroo, V. Verma, Syed Riaz-ull Hassan, R. Anand, A.P. Bhondekar, Ashwani Kumar, L.M. Bhardwaj, R.P.Bajpai. | Microbial Decontaminator | Application 3930/DELNP/2004 A, Filing date: 2004-12-10, Publication date: 2007-08-10. | 2007 |
| G.N.Qazi,S.C.Puri, G.Handa, N.Verma, R.Anand, R.K.Raine and O.P.Suri. | A novel process for isolation of hyperforin. | Patent no.: 194988 | 2007 |
| G.N.Qazi, K.L.Bedi, R.K.Johri, S.C.Sharma, M.K.Tikoo, A.K.Tikoo, S.T.Abdullah, K.Singh, R.Pandita, O.P.Suri, B.D.Gupta, K.A.Suri, N.K.Satti, R.K.Khajuria, B.K.Kapahi, A.K.Kalsotra, N.Verma, R.Anand | Bioavailabiliy/bioefficacy enhancing activity of Cumium cymium and extracts and fractions thereof. | Publication no.: WO/2004/009061, publication date: 29.01.2004, US patent no.: US 7,070.814 B2 | 2004 |
| Shaikh Tassaduq Abdullah, Pyare Lal Sangwan, Katare Anil Kumar, Kumar Amit, Nazir Ahmad Lone,Umar Ahmad Sheikh,Raghu Rai Sharma,Rajneesh Anand, Vishwakarma Ram Asrey | A Formulation of Glycyrrhiza Glabra based hydrogel product for skin Photoprotection and Preparation Thereof | Indian and International patent filled. | 2020 |
| Bharate Sonali Sandip, Singh Rohit, Gupta Mehak, Singh Bikarma, Katare Anil Kumar, Kumar Ajay, Bharate Sandip Bibishan, Vishwakarma Ram. | Gastroretentive Sustained Release Formulations of Bergenia Ciliata | International patent filled IN/16.10.17/INA201711036683 | 2018 |
 Screen Reader Access
Screen Reader Access