
Quality Control, Quality Assurance & CMC Division
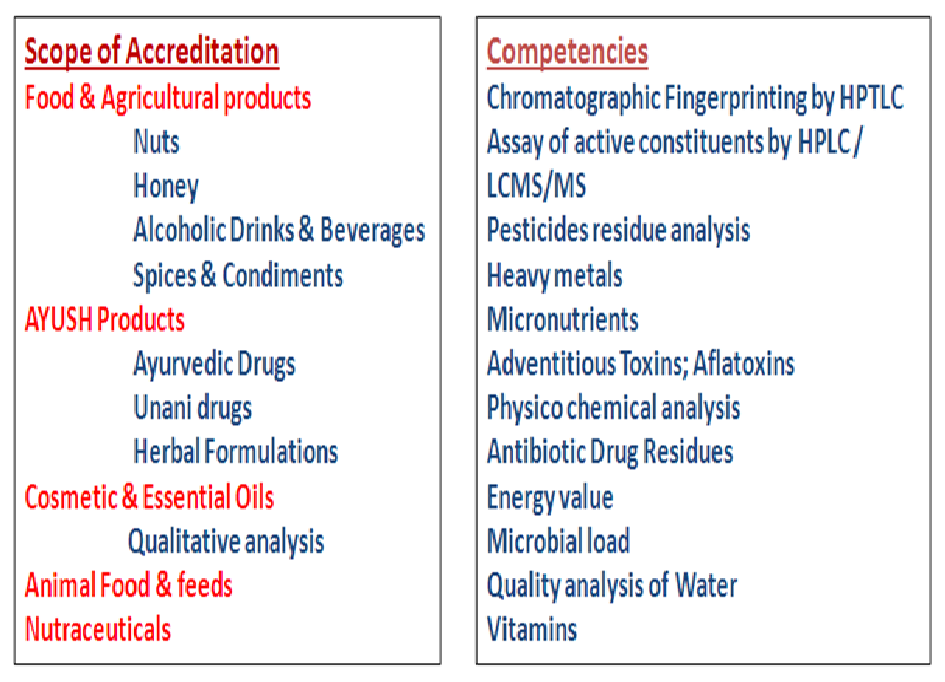
QCQA is a National Accreditation Board for Testing and Calibration Laboratories (NABL) accredited division in field of chemical testing. It is dedicated to render analytical services of highest quality meeting the requirement of the standard ISO/ IEC 17025:2017 and other criteria’s of NABL. It is the first notified Food Testing Laboratory in UT of J & K approved by GOI, Ministry of Health and Family Welfare under Section 43 (1) of FSS Act, 2006 for food commodities such as Nuts, Honey and Nutraceuticals.
QCQA Division

Certificates

Wet Lab

High Performance -Thin Layer Chromatography

Liquid Chromatography-Mass Spectrometry

Inductively Coupled Plasma Mass Spectrometry

- To deliver basic and applied research outputs of international quality in field of chemical science.
- To render analytical services of highest quality associated with degree of professional satisfaction and confidence to the customer and to meet the requirement of ISO/IEC 17025:2017 and other criteria of NABL.
- Chemistry Manufacturing & Control of Botanical Raw Material, Botanical Drug Extract and Botanical product / formulations.
- Quality Control, Evaluation and Identification for characteristic marker in Medicinal & Aromatic Plants.
- Quality Control and Assurance for authentication of Indian System of Medicine products and formulations.
- Qualitative analysis of Essential oils.
- Physicochemical analysis of Water.
- Analysis of micronutrients & heavy metals.
- Analysis of residual pesticides.
- Analysis of microbial load / pathogens in food and water.
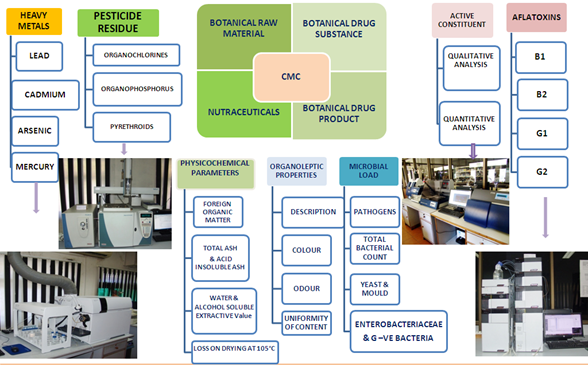
QCQA Division has facility and expertise to generate Chemistry Manufacturing & Control (CMC) data of extracts and formulations. Generation of CMC data has been executed on the following high value medicinal plants.
| Woodfordia fruticosa | Boswellia serrata | Withania somnifera |
| Colebrookea oppositifolia | Dysoxylum binecteriferum | Bergenia ciliata |
| Cocculus hirsutus | Tinospora cordifolia | Glycyrrhiza glabra |
The Division has following State of Art facilities
| S.No | Instrument | Analysis performed |
| 1 | LC-MS/MS Triple Quad | Quantitative analysis |
| 2 | ICP-MS | Heavy Metals |
| 3 | HPLC Nexera | Qualitative & Quantitative analysis |
| 4 | HPTLC | Chemical Fingerprinting |
| 5 | Kjeldahl apparatus | Estimation of Nitrogen |
1) Quality Control, Quality Assurance and Chemistry Manufacturing and Control of drugs : MLP-7001 & STS 0006.
2) Phytopharmaceutical development of Ficus semicordata as per regulatory guidelines of DCGI: GAP 2180.
| Authors | Title | Journal |
| Varun P. Singh, Anup S. Pathania, Manoj Kushwaha, Samsher Singh, Vandana Sharma, Fayaz Malik, Inshad Khan, Anil Kumar, Deepika Singh*, Ram A. Vishwakarma* | 14-Residue Peptaibol Velutibol A from Trichoderma velutinum: Its structural and cytotoxic evaluation. | RSC Advances, 2020, 10, 31233-31242. |
| Vikas Kumar, Sonali Bharate, M. Gupta, S. G. Gandhi, Deepika Singh, Sundeep Jaglan, A. Kumar, Ram A Vishwakarma, Sandip B Bharate | Evaluation of rohitukine-enriched fraction of Dysoxylum binecteriferum Hook. F. (Leaves) as Anti-arthritic Phytopharmaceutical Candidate: Chemical Standardization, In-vivo validation, Formulation development and Oral Pharmacokinetics. | Journal of Ethnopharmacology, 2020, 254, 112758. |
| Varun P Singh, Richa Sharma, Vandana Sharma, Chand Raina, Kamal K Kapoor, Anil Kumar, Asha Chaubey, Deepika Singh*, Ram A Vishwakarma* | Isolation of depsipeptides and optimization for enhanced production of valinomycin from the North-Western Himalayan cold desert strain Streptomyces lavendulae | Journal of Antibiotics, 2019, 72, 617-624 |
| I A Khan*, A Singh, D. P Mindala, S. Meena, B. Vij, A K. Yadav, Sumit Roy, Utpal Nandi, Anil K Katare, S Jaglan, Deepika Singh, A. P Gupta, Prasoon Gupta, Surjeet Singh, M. K. Tikko, G D Singh, Ram A Vishawakarma* | Preclinical development of gastro-protective botanical candidate from Woodfordia fruticosa (Linn.) Kurz: Chemical standardization, efficacy, pharmacokinetics and safety pharmacology. | Journal of Ethnopharmacology, 2019, 241, 112023. |
| Meenu Katoch*, Deepika Singh*, Kamal K Kapoor, Ram A Vishwakarma. | Trichoderma lixii (IIIM-B4), an endophyte of Bacopa monnieri L. producing peptaibols | BMC Microbiology, 2019, 19:98. |
| Sandip B. Bharate, Vikas Kumar, Sonali S. Bharate, Bikarma Singh, Gurdarshan Singh, Amarinder Singh, Mehak Gupta, Deepika Singh, Ajay Kumar, Surjeet Singh, Ram A Vishwakarma. | Discovery and preclinical development of IIIM-160, a Bergenia ciliata-based anti-inflammatory and anti-arthritic botanical drug candidate | Journal of Integrative Medicine, 2019, 17, 192-204. |
| V. P Singh, Yedukondalu N, M. Kushwaha, R. Sharma, A. Chaubey, A. Kumar, Deepika Singh*, Ram A. Vishwakarma*, | Lipopeptaibols A-D: Cytotoxic lipopeptaibols from the Himalyan Cold Habitat fungus Trichoderma velutinum | J. Nat. Prod., 2018, 81, 219-226.
|
| S. S. Bharate, V. Kumar, G. D. Singh, A. Singh, Mehak Gupta, Deepika Singh, Ajay Kumar, Ram A Vishwakarma, Sandip B. Bharate. | Preclinical Development of Crocus sativus-Based Botanical Lead IIIM-141 for Alzheimer’s Disease: Chemical Standardization, Efficacy, Formulation Development, Pharmacokinetics and Safety Pharmacology | ACS Omega, 2018, 3, 9572-9585 |
| R Sharma, Varun P Singh, Deepika Singh*, Farnaz Yusuf, Anil Kumar, Ram A Vishwakarma, Asha Chaubey | Optimization of nonribosomal peptides production by a psychrotrophic fungus: Trichoderma velutinum ACR-P1. | Applied Microbiology & Biotechnology, 2016, 100, 9091-9102 |
| R. Sharma, V. L. Jamwal, V. P. Singh, P. Awasthi, Deepika Singh, Ram A Vishwakarma, Sumit Gandhi, Asha Chaubey, | Revelation and cloning of valinomycin synthetase genes in Streptomyces lavendulae ACR-DA1 and their expression analysis under different fermentation and elicitation conditions. | Journal of Biotechnology, 2017, 253, 40-47 |
 Screen Reader Access
Screen Reader Access



